How Does Atomic Explain the Difference Between Spectral Lines
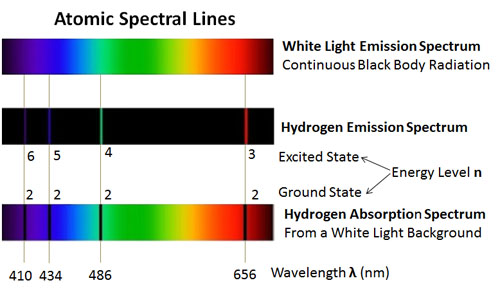
Light or visible light is electromagnetic radiation within the portion of the electromagnetic spectrum that is perceived by the human eye. Visible light is usually defined as having wavelengths in the range of 400700 nanometres nm corresponding to frequencies of 750-420 terahertz between the infrared with longer wavelengths and the ultraviolet with shorter.
The spectral analysis of the plasma emission gives access to the elemental composition of the ablated material and thus the composition of the material under study.

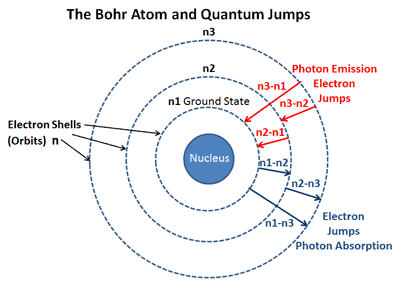
. An electron on returning from some higher energy level to the first energy level that is n 1 1 and n 2 2 3 4 etc then the emitted series of spectral lines are obtained in the ultraviolet region. 1 for the 2d case. The energy difference between 12 C and 13 C graphene is 77 meV at the highest energy peak in PDOS corresponding to the LO mode and 67 meV at the second highest energy peak which is mainly.
The LAMMPS simulation box is a 3d or 2d volume which can be orthogonal or triclinic in shape as illustrated in Fig. Orthogonal means the box edges are aligned with the x y z Cartesian axes and the box faces are thus all rectangular. As the difference between the elemental concentrations of two components endmembers approaches 0 the hyperbolas flatten to lines.
Triclinic allows for a more general parallelepiped shape in which edges are aligned with three arbitrary vectors. The hyperbolas are concave or convex depending on whether the component with the higher isotope ratio has a higher or lower concentration than the other component. Installed on an inspection robot equipped with an articulated arm the LIBS tool consists of an optical fibre carrying the incident laser light and the light emitted during the interaction of the.

No comments for "How Does Atomic Explain the Difference Between Spectral Lines"
Post a Comment